Abstract
Background: Carfilzomib (CFZ) is a next-generation proteasome inhibitor currently approved in combination with lenalidomide and dexamethasone (KRd) or with dexamethasone alone (Kd) for treatment of multiple myeloma (MM) patients with ≥1 prior line of therapy in the Asia-Pacific (APAC) region. Here, we describe utilization of KRd and Kd in routine clinical practice in APAC, including treatment patterns, patient profile, reasons for discontinuation, clinical outcomes, and frequency of adverse events (AEs).
Methods: This prospective, single-arm cohort study recruited adults with relapsed/refractory MM who received ≥1 dose of CFZ in routine clinical practice as either a combination or monotherapy regimen and had received ≥1 prior line of MM treatment before CFZ initiation. Between July 2019 and June 2021, approximately 300 patients were planned to be enrolled in this study. Medical history, patient characteristics, and clinical data are collected at baseline and throughout the 2-year observation period or until death, withdrawal of consent, or loss of follow-up. Follow-up data are collected through chart reviews every 3 months. AEs leading to treatment discontinuation are collected; common AEs associated with drugs/disease were excluded from collection by the protocol (all serious AEs were collected). The study is ongoing, and last patient record for the analysis will be collected no later than Q1 2023.
Results: As of data cutoff April 1, 2022, 311 patients were enrolled, of whom 38 are excluded from the present analysis (11 died before waiver of informed consent approval and 27 patients had missing data for the first medical chart review or first CFZ administration). The 273 patients included in this ad hoc interim analysis were enrolled from 5 countries/regions: Australia (n=46), Hong Kong (n=17), Korea (n=172), Singapore (n=13), and Taiwan (n=25). To date, the regimens prescribed were KRd (n=160, 59%), Kd (n=103, 38%), and other CFZ-based regimens (n=10, 4%; monotherapy [n=1]; not classified [1]; or with dexamethasone and cyclophosphamide [5], pomalidomide [2], or daratumumab [1]).
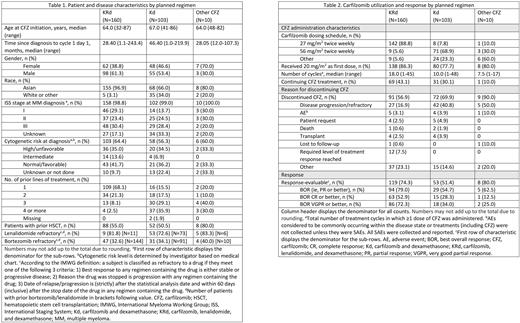
Table 1 displays detailed patient and disease characteristics, and Table 2 displays CFZ utilization and response.
Overall, 33% of patients reported a history of hypertension, 11% of cardiac disorders, 7% of diabetes mellitus, and 3% of pulmonary embolism, prior to CFZ initiation. On average, patients prescribed KRd were slightly younger than those prescribed Kd. The majority of KRd patients (68%) had received 1 prior line of treatment, whereas one-third of Kd patients (36%) had received ≥4 prior lines of treatment. Similar proportions of KRd (33%) and Kd (34%) populations were bortezomib refractory, and 73% of Kd patients were lenalidomide refractory.
The planned CFZ dose at first cycle was in accordance with the local product information and was used in 89% of KRd and 69% of Kd patients (27 and 56 mg/m2 twice weekly, respectively). At data cutoff, 43.1% and 30.1% of KRd and Kd patients, respectively, remained on CFZ treatment. The most common reason for CFZ discontinuation was disease progression/refractory disease.
With KRd and Kd, respectively, the best overall response rate was 79.0% (n=94/119) and 54.7% (n=29/53) and median overall survival was 60.9 (95% confidence interval [CI], 55.9-not estimable [NE]) and 54.5 (95% CI, 33.3-NE) months.
CFZ-treatment-related AEs of interest occurred in 10 (3.7%) total patients (cardiac arrhythmia, n=1 Kd; cardiac failure, 1 KRd; dyspnea, 2 KRd, 2 Kd, 1 other CFZ; hematopoietic thrombocytopenia, 1 KRd and 1 Kd; ischemic heart disease, 1 KRd; no incidence of treatment-related acute renal failure, hypertension, and peripheral neuropathy). Overall, 10 (3.7%) patients discontinued CFZ owing to an AE.
Conclusions: This study investigates the real-world use of CFZ for relapsed/refractory MM in a predominantly Asian patient population across the APAC region. Interim results confirm that the standard dosing schedules for KRd and Kd are well tolerated in real-world practice and suggest a very low rate of discontinuation due to CFZ-related AEs, even in heavily pretreated MM. Further prospective data collection of patients in routine practice is ongoing to assess patterns of longer-term patient management, patterns after CFZ treatment failure, CFZ toxicity according to number of previous lines of therapy, response, survival outcome, and AEs, which will inform optimal use of CFZ.
Disclosures
Quach:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding. Yoon:Astellas Pharma: Consultancy; Celgene: Consultancy; Janssen Pharmaceutical: Consultancy; Kyowa Kirin: Research Funding; Novartis: Consultancy; Amgen: Consultancy; Takeda: Consultancy; Chugai Pharmaceutical: Consultancy; Roche-Genetech: Research Funding; Yuhan Pharmaceutical: Research Funding; Tikaros: Consultancy. Kim:Janssen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; LG Chem: Membership on an entity's Board of Directors or advisory committees. Nagarajan:Janssen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau; BMS/Celgene: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ho:Janssen: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees. Low:BMS: Honoraria; Janssen: Consultancy. Huang:Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Chugai: Honoraria; Amgen: Honoraria; AbbVie: Honoraria; Alexion: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Roche: Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Wong:Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; F. Hoffmann-La Roche AG: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis Pharmaceuticals: Research Funding, Speakers Bureau. Liao:PAREXEL International (contracted to Amgen Inc): Current Employment, Current equity holder in publicly-traded company. Lin:PAREXEL International (contracted to Amgen Inc): Current Employment, Current equity holder in publicly-traded company. Britland:Amgen Inc: Current Employment, Current equity holder in publicly-traded company. Li:Amgen Inc: Current Employment, Current equity holder in publicly-traded company. Chai:Amgen Inc: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal